Case Study Question 2 on Amines – Chapter 13 CBSE Class 12 Chemistry
Case Study Question on Amines – Chapter 13 CBSE Class 12 Chemistry
Question : The conversion of primary amines into diazonium salts is known as diazotization. Arene diazonium salts are generally colourless crystalline solids highly soluble in water. These salts are more stable than aliphatic diazonium salts and undergo a number of substitution reactions due to excellent leaving ability of diazo group as N2. Arene diazonium salts also couple with phenols and amines to form coloured azo dyes. Such type of reactions are known as coupling reactions.
1. Why are arene diazonium salts more stable than aliphatic diazonium salts?
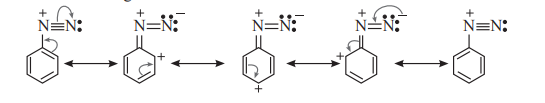
Ans. Arene diazonium salts are more stable than aliphatic diazonium salts due to dispersal of positive charge on the benzene ring as shown below.
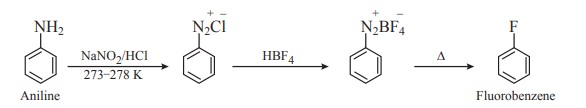
2. How will you convert: Aniline to fluorobenzene?
3. Why are benzenediazonium salts soluble in water?
Ans. Being ionic, they are soluble in water.
4. What is diazotisation?
Ans. The reaction of converting aromatic primary amines into diazonium salts by treatment with a solution of nitrous acid at 273 K–278 K is called diazotisation.
5. What product is formed when aniline is first diazotised and then reacted with phenol in the alkaline medium?
Ans. p-hydroxyazobenzene
Case Study Question 1 on Amines – Chapter 13 CBSE Class 12 Chemistry – Neutron Classes
