Short Answer Questions – 3 marks – Solutions – Chapter 2 CBSE Class 12 Chemistry
Solutions – Chapter 2 CBSE Class 12 Chemistry
Short Answer Questions – 3 marks
Q. 1. At 25°C the saturated vapour pressure of water is 3.165 kPa (23.75 mm Hg). Find the saturated vapour pressure of a 5% aqueous solution of urea (carbamide) at the same temperature. (Molar mass of urea = 60.05 g mol–1) [CBSE (F) 2012]
Ans.

Q. 2. Calculate the boiling point of solution when 2g of Na2SO4 (M = 142 g mol–1) was dissolved in 50 g of water, assuming Na2SO4 undergoes complete ionisation. (Kb for water = 0.52 K kg mol–1) [CBSE North 2016]
Ans.

Q. 3. A solution of glucose (Molar mass = 180 g mol–1) in water has a boiling point of 100.20°C. Calculate the freezing point of the same solution. Molal constants for water Kf and Kb are 1.86 K kg mol–1 and 0.512 K kg mol–1 respectively. [CBSE (F) 2017]
Ans.
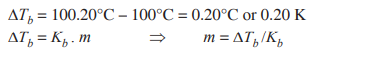
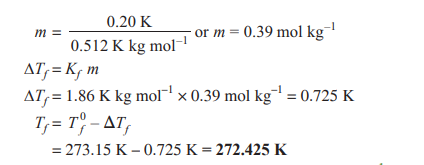
Q. 4. Calculate the freezing point of a solution when 3g of CaCl2 (M = 111 g mol–1) was dissolved in 100g of water, assuming CaCl2 undergoes complete ionisation. (Kf for water = 1.86 K kg mol–1) [CBSE East 2016]
Ans.

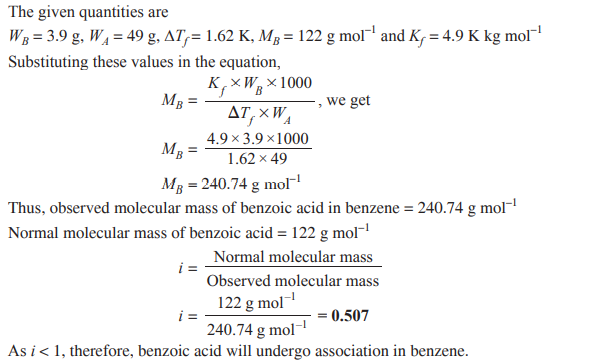
Q. 5. 3.9 g of benzoic acid dissolved in 49 g of benzene shows a depression in freezing point of 1.62 K. Calculate the van’t Hoff factor and predict the nature of solute (associated or dissociated). (Given: Molar mass of benzoic acid = 122 g mol–1, Kf for benzene = 4.9 K kg mol–1) [CBSE Delhi 2015]
Ans.
Q. 6. Calculate the mass of NaCl (molar mass = 58.5 g mol–1) to be dissolved in 37.2 g of water to lower the freezing point by 2°C, assuming that NaCl undergoes complete dissociation. (Kf for water = 1.86 K kg mol–1) [CBSE (F) 2015]
Ans.

Q. 7. At 300 K, 30 g of glucose, C6H12O6 present per litre in its solution has an osmotic pressure of 4.98 bar. If the osmotic pressure of another glucose solution is 1.52 bar at the same temperature, calculate the concentration of the other solution. [CBSE 2019 (56/4/2)]
Ans.

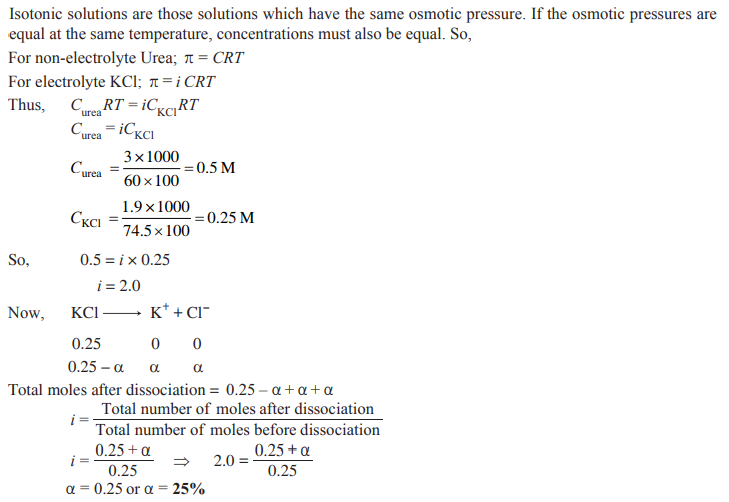
Q. 8. A solution containing 1.9 g per 100 mL of KCl (M = 74.5 g mol–1) is isotonic with a solution containing 3 g per 100 mL of urea (M = 60 g mol–1). Calculate the degree of dissociation of KCl solution. Assume that both the solutions have same temperature. [CBSE 2019 (56/2/1)]
Ans.
Also Read :
Short Answer Questions – 2 marks – Solutions – Chapter 2 CBSE Class 12 Chemistry – Neutron Classes
Very Short Answer Questions Solutions – Chapter 2 CBSE Class 12 Chemistry – Neutron Classes
